Projects
We study a diverse array of cells from the immune system (e.g., T and B lymphocytes), cardiovascular system (e.g., platelets and endothelial cells), and nervous system (e.g., neurons and astrocytes). Our work focuses on key surface receptors, including adhesion molecules (e.g., selectins and integrins), immunoreceptors (e.g., TCR, BCR, PD-1, CD40), and synaptic receptors (e.g., glutamate delta receptor and neurexin), to understand how they mediate cellular communication and mechanotransduction.
Featured
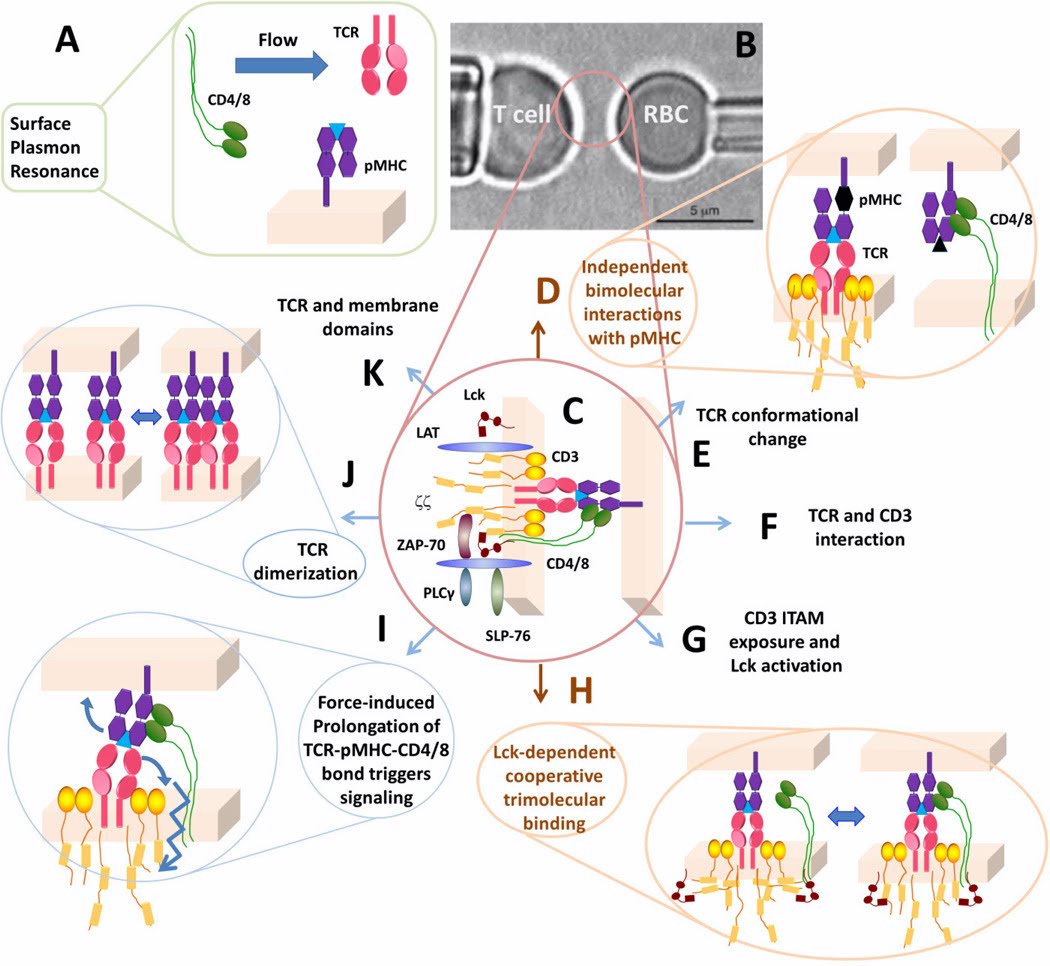
Mechanical forces on immunoreceptors emerge during migration or adhesion to cells or the ECM, and during formation of the immunological synapse. Forces exerted on specific immunoreceptor–ligand bonds potentially induce mechanotransduction. Lymphocytes use force to amplify antigen discrimination and respond to changes in substrate stiffness.
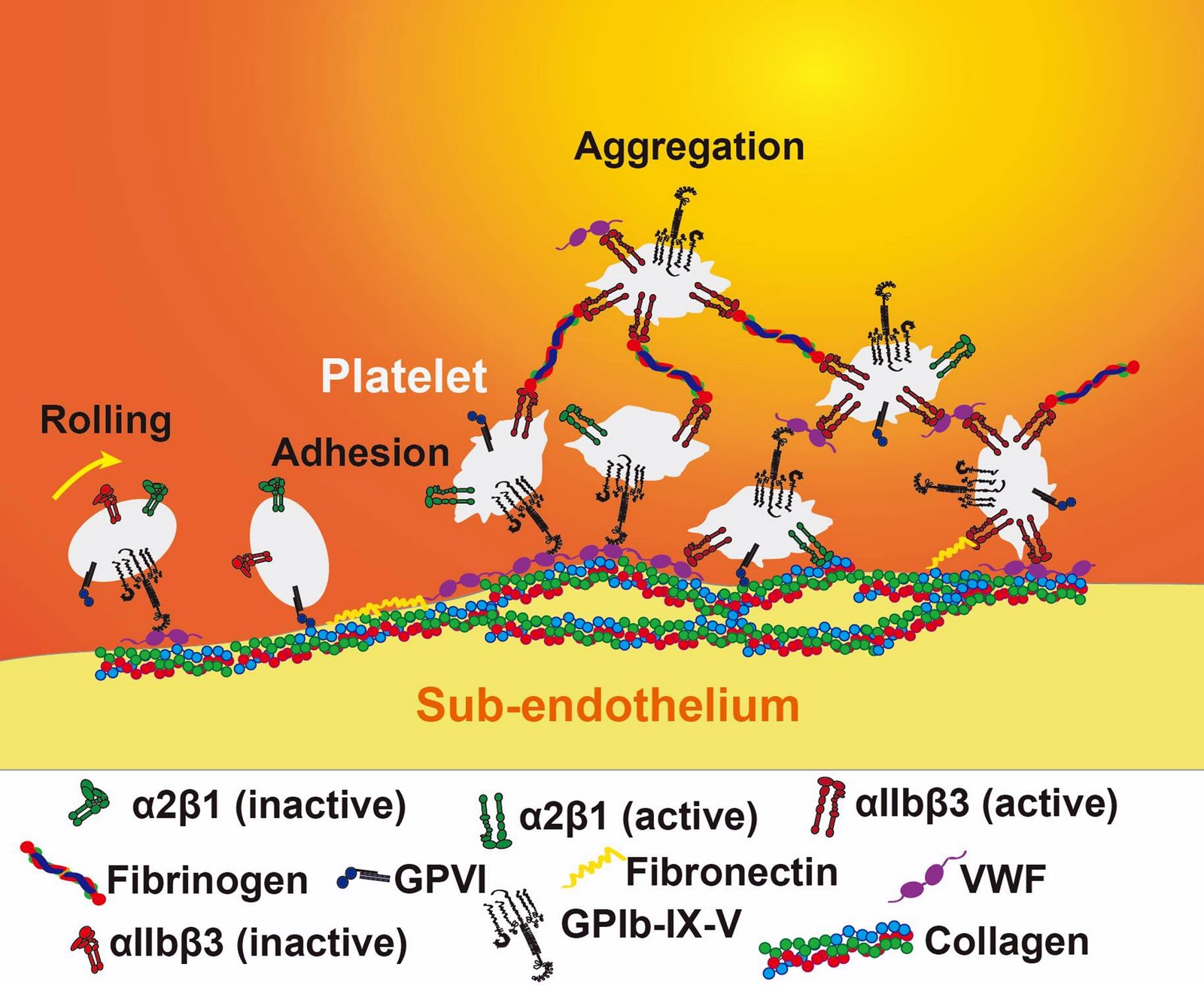
Mechanical stimuli are crucial in platelet activation in flowing blood. How platelets interpret mechanical forces along with biochemical stimuli is unclear. We have so far identified intracellular components that regulate platelet behavior in disturbed flow, which is predominantly mediated by integrins that exhibit distinct affinity and conformatio
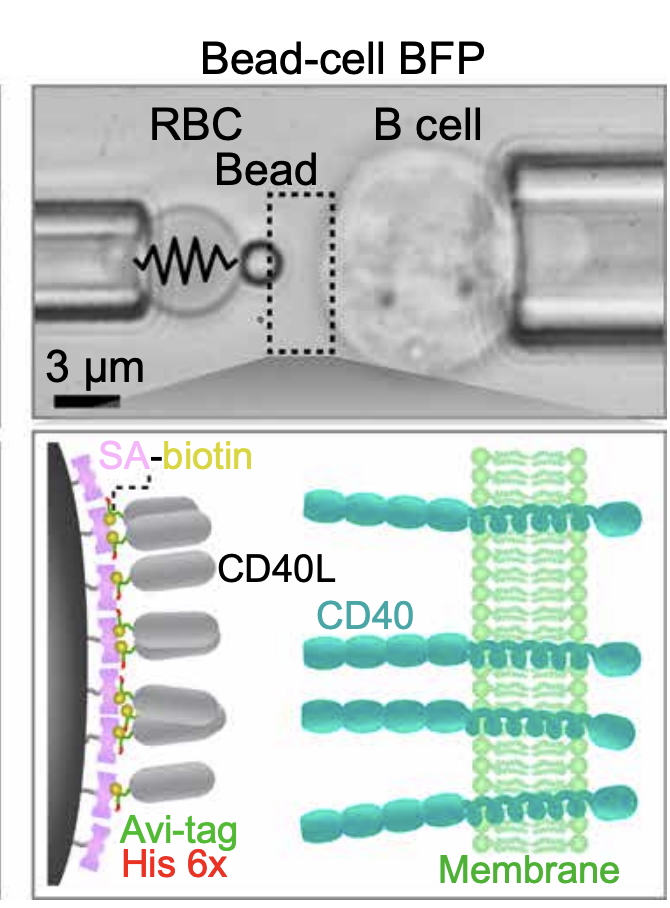
Antibody class-switch in germinal-center B cells requires signaling induced by the interaction of the B cell CD40 receptor with its ligand, CD40L (CD154), presented on CD4+ T helper cells. Rare mutations to the CD40L protein affecting its expression, binding, or function, lead to dysregulation of B cell signaling and their inability to undergo antibody class-switch. We investigate the dys-mechanoregulation of CD40–CD40L interactions by (X-linked hyper IgM)X-HIgM mutations and their effects on B cell signaling.
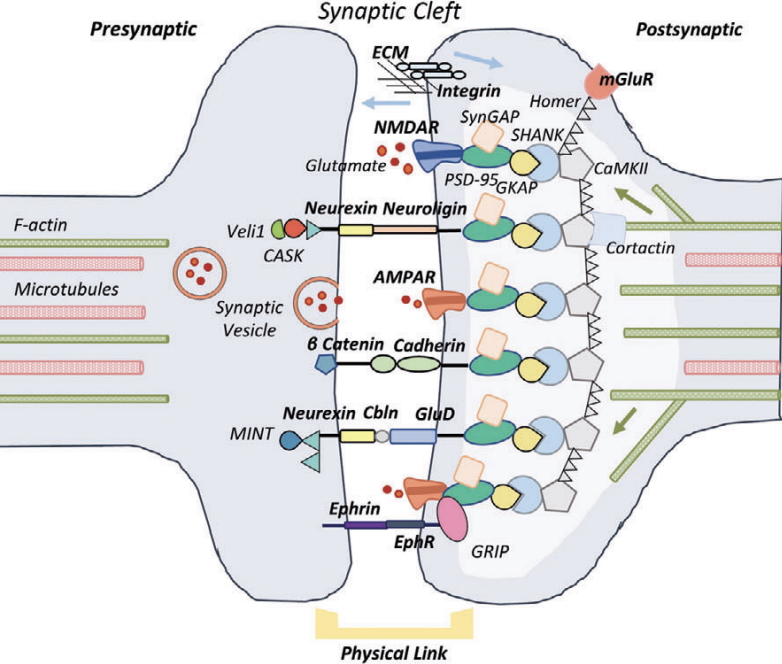
This work uncovers a mechanobiological role for the glutamate delta 1 receptor (GluD1) in synapse formation, showing that GluD1 responds to mechanical forces at the synaptic cleft through interactions with Neurexin 1β and Cerebellin 2. Using biophysical tools and neurobiological assays, the study reveals that neuronal cytoskeleton-generated forces modulate GluD1 binding, signaling, and synaptogenesis. These findings position GluD1 as a mechanosensitive receptor, with its force-regulated behavior altered by neuromodulators and disease-linked mutations—establishing a new paradigm in neuronal mechanotransduction.